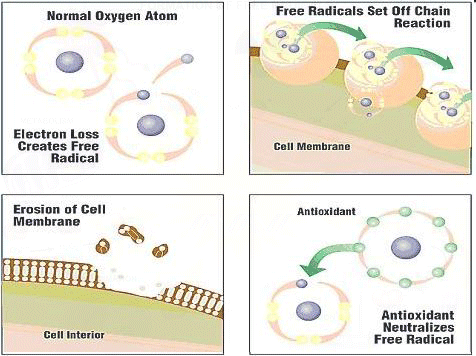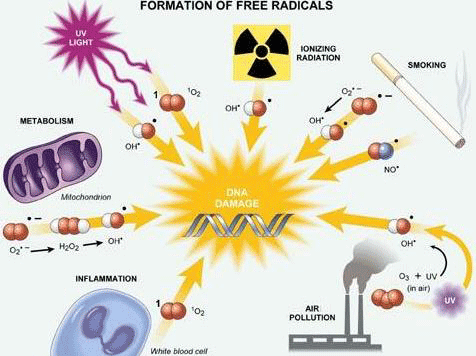
Free Radicals Antioxidants are substances that have the ability to counteract the damaging effects of oxidation. Free radicals are chemically active atoms that have a charge due to an excess or deficient number of electrons. When an excessive amount of free radicals form beyond the capacity the antioxidant defenses in a person's body can handle, oxidative stress occurs. Free radicals have unpaired electrons, meaning that they are highly unstable. As a result, free radicals search inside a person's body to grab or donate electrons to reach stability. This results in the damage of cells, proteins, and DNA. Since free radicals come from both inside and outside sources than our bodies, it is nearly impossible to avoid this damage. Within our bodies, oxidants come from processes of aerobic respiration, inflammation, and metabolism. Free radicals from outside sources include many different environmental factors such as exercise, pollution, sunlight, X-rays, smoking, as well as alcohol. Cell parts that have been damaged by oxidation will accumulate, since the antioxidant defenses in our body will disintegrate as we age. (Citation 8) The Antioxidant Process First, antioxidants attack the oxidation process by becoming oxidized. This process is when antioxidants neutralize free radicals. The antioxidant process can be classified in two ways:
|
Oxidation Oxidation, a natural process, is disturbed when free radicals form and attract to other molecules in our bodies. Oxidative stress is an evident cause of aging and is the reason for many skin imperfections. Oxidative stress in our bodies can cause diseases such as heart disease, cancer, arthritis, lung disease, fibromyalgia, diabetes, Parkinson’s, Alzheimer’s, autoimmune diseases, and eye diseases. (Citation 18) Enzymes play a fundamental role in catalyzing the process of oxidation. For example, Cytochrome P450, a family of oxidative enzymes, are believed to metabolize compounds such as carcinogens and drugs. (Citation 19) Fatty acid oxidation is the process of fatty acids being oxidized to become energy in our bodies. Common fatty acids are triglycerides, which are mainly in vegetable oils and animal fats. To speed up the process of fatty acid oxidation, many people take vitamin supplements or eat plenty of Omega 3 Fatty Acids. Foods such as fish, eggs, milk, and cheese with Omega 3 Fatty Acids are proven to speed up one's metabolism. (Citation 20) |
|